Garni Barkhoudarian1, Julian P. Whitelegge2, Daniel F. Kelly1, Margaret Simonian2
1. John Wayne Cancer Institute, Providence St John’s Health Center, USA
2. David Geffen School of Medicine, University of California Los Angeles (UCLA), USA
Bio:

Dr. Margaret Simonian has MPhil and PhD in Advanced Medicine. Works as a Research Scientist/Specialist at UCLA, David Geffen School of Medicine, previously worked as a Senior Research Fellow and Lecturer, at LA-Biomed Research Institute, Harbor-UCLA, and Macquarie University, Macquarie Neurosurgery (Australia). Her research interest focuses on utilizing Proteomics and Molecular Biology in biomarker discovery and drug development/therapy of diseases, as well as advancing cancer immunotherapy with Proteomics approaches, and Radioproteomics for cancer treatment. She is a recognised Reviewer & Editorial board member for multiple scientific journals and a member of (B/D-HPP).
Article:
Meningiomas are the most common benign intracranial tumors and their first-line treatment is surgical removal if the lesion can be largely removed at sufficiently low risk. However, a subset of patients develops more aggressive tumors. According to the World Health Organization (WHO), meningiomas are classified as typical (1), atypical (ll) and anaplastic (lll); up to 20% of patients may have atypical meningiomas and 1-3% may develop anaplastic or malignant subtypes [1]. These aggressive subtypes of tumors typically exhibit more rapid tumor progression, invasiveness and recurrence precluding complete surgical removal and requiring additional therapies of radiosurgery/radiotherapy and chemotherapy [2]. Occasionally, meningiomas have malignant transformation with distant metastases outside the central nervous system (CNS).
Few genetic and proteomics markers have been studied for meningioma subtypes with various aims [3,4] and their correlation to clinical behaviour and response to therapy is limited. While there is a notable overlap with some biomarkers found in other malignant neoplasms (glioblastoma, adenocarcinoma, squamous cell carcinoma and melanoma), the mechanisms that result in transformation from benign meningiomas to more aggressive subtypes are poorly understood.
Hence, this study aimed to better define biomarkers of transformation into the aggressive/malignant subtypes, and to identify targets for future therapies. Three tumor subtype (typical, atypical and anaplastic), and control (fresh cadaveric dura) tissues were used for the proteomics analysis. Multiplex peptide stable isotope labelling method was used to label all samples. With this method, all primary amines (the N terminus and the side chain of lysine residues) in a peptide mixture are converted to dimethylamines. The labelled samples are then mixed in equal ratios and analysed by liquid chromatography–mass spectrometry (LC/MS). The mass difference of the dimethyl labels is used to compare the peptide quantity across all samples.
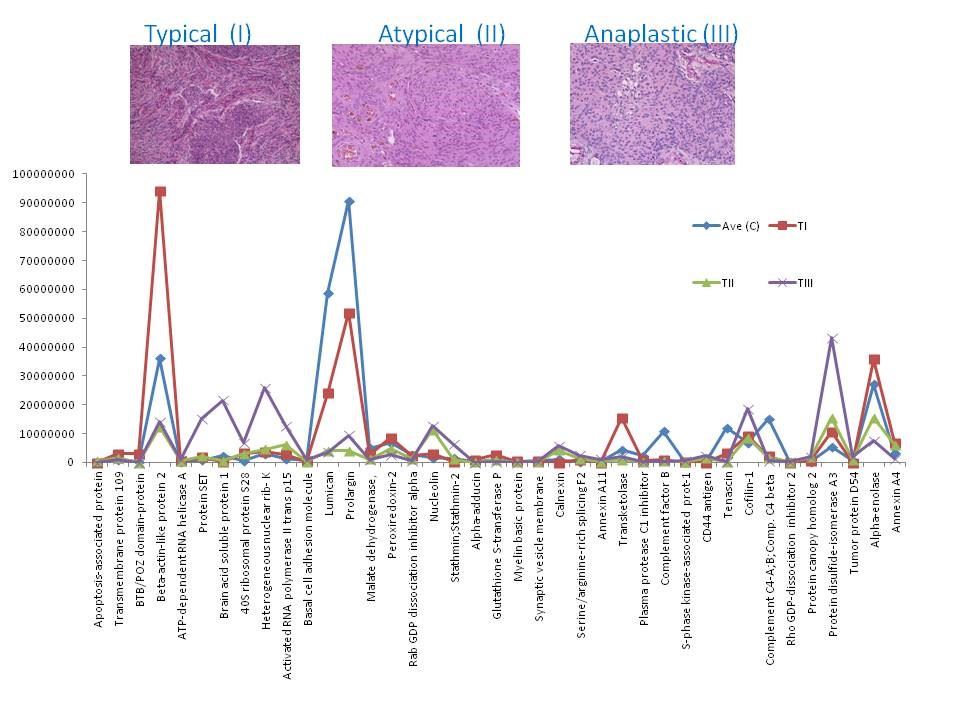
Our analysis and observation was focused on the proteins that showed up or down-regulation in one phenotype compared to the others and compare to the control, as those proteins could potentially be investigated as biomarkers for aggressive tumors, e.g. protein alpha-adducin, was expressed in C, I and II only, and it was up-regulated in grade I by 3 fold compare to the control, however in II was down-regulated by 0.25 compare to the control, and wasn’t detected in III; hence the expression ratio for (I : II) was 11.6 fold. This may suggest that this protein is mainly present in the non-aggressive form of meningioma, or its representing gene (ADD1) may be switched off in the aggressive forms. Many other proteins showed similar pattern (Fig 1). Another intriguing observation of this data was the presence of some proteins in one subtype only compare to other subtypes and compare to the control. Twenty three proteins were detected in grade III only, including tumor protein D52, lysosome membrane protein 2, splicing factor-1 and MUC18. These proteins are of importance in biomarker study of meningiomas due to their unique expression. The full data is available in PubMed [5].
This data suggests the feasibility of identifying and quantifying the proteins in brain minengioma tissues for comparison studies. We are obtaining larger numbers of specimen to conduct a large scale experiments to significantly identify novel protein biomarkers that correlate with the aggressive tumors. These biomarkers will be clinically utilized in future management of patients, to better identify aggressive tumors for closer surveillance and application of novel targeted therapies. Ultimately this may potentially reduce the need for major high-risk surgery in this patient population.
Figure 1: Chart of the expression levels of selected proteins from. Their intensities in control (C) and meningioma tissues grade (I, II and III).
References:
1. Commins DL, Atkinson RD, Burnett ME (2007) Review of meningioma histopathology. Neurosurg Focus 23: E3.
2. Doleželová H, Hynková L, Pospíšil P, Kazda T, Slampa P, et al. (2012) Therapeutic results of the treatment brain tumors using radiosurgery and stereotactic radiotherapy. Klin Onkol 25: 445-451
3. Lusis EA, Chicoine MR, Perry A (2005) High throughput screening of meningioma biomarkers using a tissue microarray. J Neurooncol 73: 219-223.
4. Sharma S, Ray S, Moiyadi A, Sridhar E, Srivastava S (2014) Quantitative proteomic analysis of meningiomas for the identificationof surrogate protein markers. Sci Rep 4: 7140.
5. Barkhoudarian G, Whitelegge JP, Kelly DF, Simonian M (2016) Proteomics Analysis of Brain Meningiomas in Pursuit of Novel Biomarkers of the Aggressive Behavior. J Proteomics Bioinform 9: 053-057.


.png)